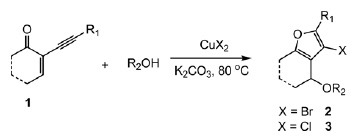
An Efficient Synthesis of Substituted Furans by Cupric Halide-Mediated Intramolecular Halocyclization of 2-(1-Alkynyl)-2-alken-1-ones
Vol. 34, No. 3, pp. 887-891, Mar. 2013
 10.5012/bkcs.2013.34.3.887
10.5012/bkcs.2013.34.3.887
Tumbnail
Abstract
Statistics
Cumulative Counts from November, 2022
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
|
|
Cite this article
[IEEE Style]
W. Fu, F. Xu, W. Guo, M. Zhu, C. Xu, "An Efficient Synthesis of Substituted Furans by Cupric Halide-Mediated Intramolecular Halocyclization of 2-(1-Alkynyl)-2-alken-1-ones," Bulletin of the Korean Chemical Society, vol. 34, no. 3, pp. 887-891, 2013. DOI: 10.5012/bkcs.2013.34.3.887.
[ACM Style]
Wei-jun Fu, Feng-juan Xu, Wen-bo Guo, Mei Zhu, and Chen Xu. 2013. An Efficient Synthesis of Substituted Furans by Cupric Halide-Mediated Intramolecular Halocyclization of 2-(1-Alkynyl)-2-alken-1-ones. Bulletin of the Korean Chemical Society, 34, 3, (2013), 887-891. DOI: 10.5012/bkcs.2013.34.3.887.

