Substituent Effects on Conformational Changes in (+)-CSA Doped Polyaniline Derivatives
Vol. 34, No. 7, pp. 2111-2116, Jul. 2013
 10.5012/bkcs.2013.34.7.2111
10.5012/bkcs.2013.34.7.2111
Tumbnail
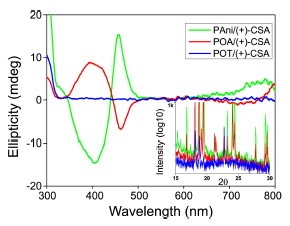
Abstract
Statistics
Cumulative Counts from November, 2022
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
|
|
Cite this article
[IEEE Style]
E. Lee and E. Kim, "Substituent Effects on Conformational Changes in (+)-CSA Doped Polyaniline Derivatives," Bulletin of the Korean Chemical Society, vol. 34, no. 7, pp. 2111-2116, 2013. DOI: 10.5012/bkcs.2013.34.7.2111.
[ACM Style]
Eung Lee and Eunok Kim. 2013. Substituent Effects on Conformational Changes in (+)-CSA Doped Polyaniline Derivatives. Bulletin of the Korean Chemical Society, 34, 7, (2013), 2111-2116. DOI: 10.5012/bkcs.2013.34.7.2111.

