Highly Efficient Synthesis of Conformationally Fixed Bicyclo[3.1.0]hexyl Nucleosides with an Ethenyl Group at C3′-Position as Potential Antiviral Agents
-
Seong Jin Kim Youngwoo Woo Ah-Young Park Hye Rim Kim Sujin Son Hwi Young Yun Pusoon Chun Hyung Ryong Moon
Vol. 35, No. 9, pp. 2649-2654, Sep. 2014
 10.5012/bkcs.2014.35.9.2649
10.5012/bkcs.2014.35.9.2649
-
5-Methylbicyclo[3.1.0]hexanone Carbenoid cycloaddition 3′-Ethenyl, North conformation Mitsunobu reaction
Tumbnail
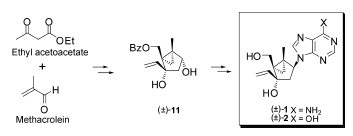
Abstract
Statistics
Cumulative Counts from November, 2022
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
|
|
Cite this article
[IEEE Style]
S. J. Kim, Y. Woo, A. Park, H. R. Kim, S. Son, H. Y. Yun, P. Chun, H. R. Moon, "Highly Efficient Synthesis of Conformationally Fixed Bicyclo[3.1.0]hexyl Nucleosides with an Ethenyl Group at C3′-Position as Potential Antiviral Agents," Bulletin of the Korean Chemical Society, vol. 35, no. 9, pp. 2649-2654, 2014. DOI: 10.5012/bkcs.2014.35.9.2649.
[ACM Style]
Seong Jin Kim, Youngwoo Woo, Ah-Young Park, Hye Rim Kim, Sujin Son, Hwi Young Yun, Pusoon Chun, and Hyung Ryong Moon. 2014. Highly Efficient Synthesis of Conformationally Fixed Bicyclo[3.1.0]hexyl Nucleosides with an Ethenyl Group at C3′-Position as Potential Antiviral Agents. Bulletin of the Korean Chemical Society, 35, 9, (2014), 2649-2654. DOI: 10.5012/bkcs.2014.35.9.2649.

