Cyanide- and Phenoxo-Bridged Heterobimetallic Fe(III)-Mn(III) Coordination Polymer: Synthesis, Crystal Structures and Magnetic Properties
Vol. 35, No. 9, pp. 2684-2688, Sep. 2014
 10.5012/bkcs.2014.35.9.2684
10.5012/bkcs.2014.35.9.2684
Tumbnail
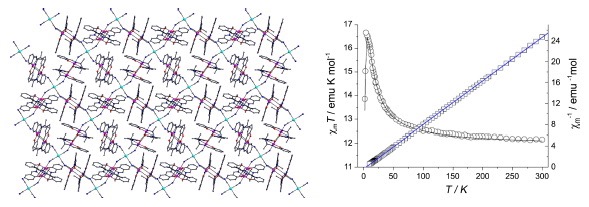
Abstract
Statistics
Cumulative Counts from November, 2022
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
|
|
Cite this article
[IEEE Style]
D. Zhang, L. Kong, Y. Li, P. Wang, X. Chen, "Cyanide- and Phenoxo-Bridged Heterobimetallic Fe(III)-Mn(III) Coordination Polymer: Synthesis, Crystal Structures and Magnetic Properties," Bulletin of the Korean Chemical Society, vol. 35, no. 9, pp. 2684-2688, 2014. DOI: 10.5012/bkcs.2014.35.9.2684.
[ACM Style]
Daopeng Zhang, Lingqian Kong, Yueyun Li, Ping Wang, and Xia Chen. 2014. Cyanide- and Phenoxo-Bridged Heterobimetallic Fe(III)-Mn(III) Coordination Polymer: Synthesis, Crystal Structures and Magnetic Properties. Bulletin of the Korean Chemical Society, 35, 9, (2014), 2684-2688. DOI: 10.5012/bkcs.2014.35.9.2684.

