Synthesis of Organic Carbonates with Alkyl/aryl 4,5-dichloro-6-oxopyridazine-1(6H)- carboxylates and ROH/AlCl3 under Ambient Condition
Vol. 35, No. 9, pp. 2758-2764, Sep. 2014
 10.5012/bkcs.2014.35.9.2758
10.5012/bkcs.2014.35.9.2758
-
Organic carbonate Alkoxide equivalent Alcohol-aluminum adduct Alkyl/aryl 4,5-dichloro-6- oxopyridazine-1(6H)-carboxylates 4,5-Dichloropyridazin-3(2H)-one
Tumbnail
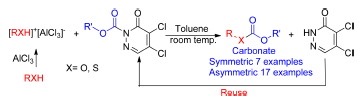
Abstract
Statistics
Cumulative Counts from November, 2022
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
|
|
Cite this article
[IEEE Style]
G. H. Sung, B. R. Kim, K. E. Ryu, J. Kim, Y. Yoon, "Synthesis of Organic Carbonates with Alkyl/aryl 4,5-dichloro-6-oxopyridazine-1(6H)- carboxylates and ROH/AlCl3 under Ambient Condition," Bulletin of the Korean Chemical Society, vol. 35, no. 9, pp. 2758-2764, 2014. DOI: 10.5012/bkcs.2014.35.9.2758.
[ACM Style]
Gi Hyeon Sung, Bo Ram Kim, Ki Eun Ryu, Jeum-Jong Kim, and Yong-Jin Yoon. 2014. Synthesis of Organic Carbonates with Alkyl/aryl 4,5-dichloro-6-oxopyridazine-1(6H)- carboxylates and ROH/AlCl3 under Ambient Condition. Bulletin of the Korean Chemical Society, 35, 9, (2014), 2758-2764. DOI: 10.5012/bkcs.2014.35.9.2758.

