Kinetics and Mechanism of Anilinolyses of Aryl Methyl and Aryl Propyl Chlorothiophosphates in Acetonitrile†
Vol. 35, No. 9, pp. 2797-2802, Sep. 2014
 10.5012/bkcs.2014.35.9.2797
10.5012/bkcs.2014.35.9.2797
-
Thiophosphoryl transfer reaction Anilinolysis Y-Aryl methyl and Y-aryl propyl chlorothiophosphates Deuterium kinetic isotope effect Cross-interaction constant
Tumbnail
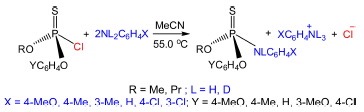
Abstract
Statistics
Cumulative Counts from November, 2022
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
Multiple requests among the same browser session are counted as one view. If you mouse over a chart, the values of data points will be shown.
|
|
Cite this article
[IEEE Style]
H. R. Barai and H. W. Lee, "Kinetics and Mechanism of Anilinolyses of Aryl Methyl and Aryl Propyl Chlorothiophosphates in Acetonitrile†," Bulletin of the Korean Chemical Society, vol. 35, no. 9, pp. 2797-2802, 2014. DOI: 10.5012/bkcs.2014.35.9.2797.
[ACM Style]
Hasi Rani Barai and Hai Whang Lee. 2014. Kinetics and Mechanism of Anilinolyses of Aryl Methyl and Aryl Propyl Chlorothiophosphates in Acetonitrile†. Bulletin of the Korean Chemical Society, 35, 9, (2014), 2797-2802. DOI: 10.5012/bkcs.2014.35.9.2797.

